Intended for US residents only
Jakafi is a prescription medicine used to treat adults with polycythemia vera who have already taken a medicine called hydroxyurea (HU) and it did not work well enough or they could not tolerate it.
Jakafi® (ruxolitinib) (JAK-ah-fye) is a prescription medicine available as a pill. It is used to treat adults with polycythemia vera
(polly-sigh-THEE-me-ah VAIR-ah), or PV, who have already taken a medicine called hydroxyurea and it did not work well enough or they could not tolerate it.
Jakafi is the first medicine approved by the Food and Drug Administration (FDA) for the treatment of these patients.
Jakafi is not chemotherapy. It is a targeted treatment that works to help keep the production of blood cells under control.
Explore possible benefits
Discover what may be possible with Jakafi for the treatment of polycythemia vera in patients who did not benefit from HU.
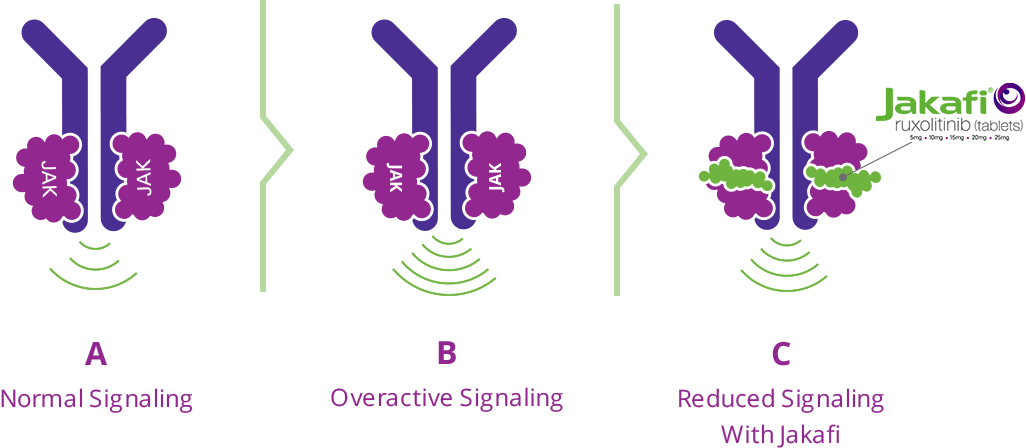
Polycythemia vera (PV) is a complex condition, and researchers are still trying to discover its exact cause. Evidence suggests that proteins known as Janus kinases, or JAKs, are involved. JAK proteins send signals that affect the production of blood cells in the bone marrow (A). When JAKs send too many signals, they cause the body to make the wrong number of blood cells. This chain of events is called overactive JAK signaling (B).
JAK signaling may become overactive in many ways. One way is by a JAK mutation. A mutation is a small change in the DNA that causes proteins to act differently than normally intended. All people with PV are believed to have overactive JAK signaling even if they do not have a JAK mutation.
Jakafi is a targeted therapy that works by targeting JAKs, which control the production of blood cells. In doing so, Jakafi helps to reduce overactive JAK signaling to help keep the production of blood cells under control (C). Jakafi may also help to reduce the size of an enlarged spleen.
Having an abnormal number of blood cells may cause your spleen to work harder than normal. This may cause the spleen to get bigger. Learn more about spleen enlargement in PV
Professional Perspective:
Exploring a Targeted Therapy Called Jakafi
Discover how Jakafi works to reduce overactive JAK signaling.
Jakafi can cause serious side effects, including:
Low blood counts: Jakafi® (ruxolitinib) may cause low platelet, red blood cell, and white blood cell counts. If you develop bleeding, stop taking Jakafi and call your healthcare provider. Your healthcare provider will do a blood test to check your blood counts before you start Jakafi and regularly during your treatment. Your healthcare provider may change your dose of Jakafi or stop your treatment based on the results of your blood tests. Tell your healthcare provider right away if you develop or have worsening symptoms such as unusual bleeding, bruising, tiredness, shortness of breath, or a fever.
Infection: You may be at risk for developing a serious infection during treatment with Jakafi. Tell your healthcare provider if you develop any of the following symptoms of infection: chills, nausea, vomiting, aches, weakness, fever, painful skin rash or blisters.
Cancer: Some people have had certain types of non-melanoma skin cancers during treatment with Jakafi. Your healthcare provider will regularly check your skin during your treatment with Jakafi. Tell your healthcare provider if you develop any new or changing skin lesions during treatment with Jakafi.
Increases in cholesterol: You may have changes in your blood cholesterol levels during treatment with Jakafi. Your healthcare provider will do blood tests to check your cholesterol levels about every 8 to 12 weeks after you start taking Jakafi, and as needed.
Increased risk of major cardiovascular events such as heart attack, stroke or death in people who have cardiovascular risk factors and who are current or past smokers while using another JAK inhibitor to treat rheumatoid arthritis: Get emergency help right away if you have any symptoms of a heart attack or stroke while taking Jakafi, including: discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back, severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw, pain or discomfort in your arms, back, neck, jaw, or stomach, shortness of breath with or without chest discomfort, breaking out in a cold sweat, nausea or vomiting, feeling lightheaded, weakness in one part or on one side of your body, slurred speech
Increased risk of blood clots: Blood clots in the veins of your legs (deep vein thrombosis, DVT) or lungs (pulmonary embolism, PE) have happened in people taking another JAK inhibitor for rheumatoid arthritis and may be
life-threatening. Tell your healthcare provider right away if you have any signs and symptoms of blood clots during treatment with Jakafi, including: swelling, pain, or tenderness in one or both legs, sudden, unexplained chest or upper back pain, shortness of breath or difficulty breathing
Possible increased risk of new (secondary) cancers: People who take another JAK inhibitor for rheumatoid arthritis have an increased risk of new (secondary) cancers, including lymphoma and other cancers. People who smoke or who smoked in the past have an added risk of new cancers.
The most common side effects of Jakafi include: for certain types of myelofibrosis (MF) and polycythemia vera (PV) – low platelet or red blood cell counts, bruising, dizziness, headache, and diarrhea; for acute GVHD – low platelet counts, low red or white blood cell counts, infections, and swelling; and for chronic GVHD – low red blood cell or platelet counts and infections including viral infections.
These are not all the possible side effects of Jakafi. Ask your pharmacist or healthcare provider for more information. Call your doctor for medical advice about side effects.
Before taking Jakafi, tell your healthcare provider about: all the medications, vitamins, and herbal supplements you are taking and all your medical conditions, including if you have an infection, have or had low white or red blood cell counts, have or had tuberculosis (TB) or have been in close contact with someone who has TB, had shingles (herpes zoster), have or had hepatitis B, have or had liver or kidney problems, are on dialysis, have high cholesterol or triglycerides, had cancer, are a current or past smoker, had a blood clot, heart attack, other heart problems or stroke, or have any other medical condition. Take Jakafi exactly as your healthcare provider tells you. Do not change your dose or stop taking Jakafi without first talking to your healthcare provider.
Women should not take Jakafi while pregnant or planning to become pregnant. Do not breastfeed during treatment with Jakafi and for 2 weeks after the final dose.
Please see the Full Prescribing Information, which includes a more complete discussion of the risks associated with Jakafi.


Register for the Incyte Mentor Program and connect with another patient taking Jakafi.
Learn HowInterested in becoming an Incyte Mentor?
Learn about the Ambassadors for Jakafi program.
Jakafi is a prescription medicine used to treat adults with polycythemia vera who have already taken a medicine called hydroxyurea and it did not work well enough or they could not tolerate it.
Jakafi is used to treat adults with certain types of myelofibrosis.
Jakafi is used to treat adults and children 12 years of age and older with acute graft-versus-host disease (GVHD) who have taken corticosteroids and they did not work well enough.
Jakafi is also used to treat adults and children 12 years of age and older with chronic GVHD who have taken one or two types of treatments and they did not work well enough.
Jakafi can cause serious side effects, including:
Low blood counts: Jakafi® (ruxolitinib) may cause low platelet, red blood cell, and white blood cell counts. If you develop bleeding, stop taking Jakafi and call your healthcare provider. Your healthcare provider will do a blood test to check your blood counts before you start Jakafi and regularly during your treatment. Your healthcare provider may change your dose of Jakafi or stop your treatment based on the results of your blood tests. Tell your healthcare provider right away if you develop or have worsening symptoms such as unusual bleeding, bruising, tiredness, shortness of breath, or a fever.
Infection: You may be at risk for developing a serious infection during treatment with Jakafi. Tell your healthcare provider if you develop any of the following symptoms of infection: chills, nausea, vomiting, aches, weakness, fever, painful skin rash or blisters.
Cancer: Some people have had certain types of non-melanoma skin cancers during treatment with Jakafi. Your healthcare provider will regularly check your skin during your treatment with Jakafi. Tell your healthcare provider if you develop any new or changing skin lesions during treatment with Jakafi.
Increases in cholesterol: You may have changes in your blood cholesterol levels during treatment with Jakafi. Your healthcare provider will do blood tests to check your cholesterol levels about every 8 to 12 weeks after you start taking Jakafi, and as needed.
Increased risk of major cardiovascular events such as heart attack, stroke or death in people who have cardiovascular risk factors and who are current or past smokers while using another JAK inhibitor to treat rheumatoid arthritis: Get emergency help right away if you have any symptoms of a heart attack or stroke while taking Jakafi, including: discomfort in the center of your chest that lasts for more than a few minutes, or that goes away and comes back, severe tightness, pain, pressure, or heaviness in your chest, throat, neck, or jaw, pain or discomfort in your arms, back, neck, jaw, or stomach, shortness of breath with or without chest discomfort, breaking out in a cold sweat, nausea or vomiting, feeling lightheaded, weakness in one part or on one side of your body, slurred speech.
Increased risk of blood clots: Blood clots in the veins of your legs (deep vein thrombosis, DVT) or lungs (pulmonary embolism, PE) have happened in people taking another JAK inhibitor for rheumatoid arthritis and may be life-threatening. Tell your healthcare provider right away if you have any signs and symptoms of blood clots during treatment with Jakafi, including: swelling, pain, or tenderness in one or both legs, sudden, unexplained chest or upper back pain, shortness of breath or difficulty breathing.
Possible increased risk of new (secondary) cancers: People who take another JAK inhibitor for rheumatoid arthritis have an increased risk of new (secondary) cancers, including lymphoma and other cancers. People who smoke or who smoked in the past have an added risk of new cancers.
The most common side effects of Jakafi include: for certain types of myelofibrosis (MF) and polycythemia vera (PV) – low platelet or red blood cell counts, bruising, dizziness, headache, and diarrhea; for acute GVHD – low platelet counts, low red or white blood cell counts, infections, and swelling; and for chronic GVHD – low red blood cell or platelet counts and infections including viral infections.
These are not all the possible side effects of Jakafi. Ask your pharmacist or healthcare provider for more information. Call your doctor for medical advice about side effects.
Before taking Jakafi, tell your healthcare provider about: all the medications, vitamins, and herbal supplements you are taking and all your medical conditions, including if you have an infection, have or had low white or red blood cell counts, have or had tuberculosis (TB) or have been in close contact with someone who has TB, had shingles (herpes zoster), have or had hepatitis B, have or had liver or kidney problems, are on dialysis, have high cholesterol or triglycerides, had cancer, are a current or past smoker, had a blood clot, heart attack, other heart problems or stroke, or have any other medical condition. Take Jakafi exactly as your healthcare provider tells you. Do not change your dose or stop taking Jakafi without first talking to your healthcare provider.
Women should not take Jakafi while pregnant or planning to become pregnant. Do not breastfeed during treatment with Jakafi and for 2 weeks after the final dose.
Please see the Full Prescribing Information, which includes a more complete discussion of the risks associated with Jakafi.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
You may also report side effects to Incyte Medical Information at 1-855-463-3463.